Bf3 Which Atom Does Not Follow Octet Rule
Sulfur and phosphorus are common examples of this behavior. Octet rule Less common than hypervalent compounds but by no means rare are species in which an atom does not achieve an octet of electrons.

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
Both A and C C.
. Both Br and F have seven valence electrons so the lewis structure will have a total of 28 electrons or 14 electron pairs. Violation of the Octet Rule for Period 3 and up or is it down based on the Periodic Table is because of the presence of d-Orbitals and usually the valency is in excess of 8 if I remember correctly. Hope it Top answer.
In addition BF3 will react with ammonia NH3 to. The rule is applicable to the main- group elements especially carbon nitrogen oxygen and the halogens but also to metals such as sodium and magnesium. The two elements that most commonly fail to complete an octet are boron and aluminum.
The octet rule is not valid for d CO. Which of the following molecules include a central atom that does not obey the octet rule. BF3 and PF5 are examples of violations of the octet rule.
Why does bf3 not follow the octet rule formal charge. An example is the compound boron trifluoride BF3 which is used as an industrial. However this puts a formal positive charge on an F atom.
BrF3 does not follow the octet rule. Jee main update Q. The electrons from both atoms travel within the molecular orbital.
Which one of the following species has a central atom that does NOT obey the octet rule. However compounds in which boron or aluminum atoms form five bonds are never observed so we must conclude that simple predictions based on the octet rule are not reliable for Group III. It only has six electrons around it.
Such compounds are called incomplete-octet compounds. But in case of nitrogen we know that it contains five electrons in its valence shell and oxygen possesses six electrons in its valence resulting in an odd electron molecule thus the octet rule is not followed in the NO molecule. A well-known example is BF 3.
Additionally which follows octet rule. Each line around the atoms represents a pair of electrons. After forming a molecule boron has only six electrons Ie three from chlorine atom and three of its own.
Which one of the following species has a central atom that does NOT obey the octet rule. Correct option is A BF 3. The problem with this structure is that boron has an incomplete octet.
They both readily form compounds in which they have six valence electrons rather than the usual eight predicted by the octet rule. In above structure F completes its octet by sharing one electron from Boron While Boron shares three electron from three F atom and has only 6 electrons in outermost cell thus it has an incomplete octet. BF4- and BF3.
Chemistry questions and answers. 3 lone electron pairs will surround each F atom and 2 lone electron pairs will be on the Br atom. What elements follow the octet rule.
The Lewis structure of which of the following compounds does not follow the octet rule. Here the central atom does not follow the octet rule. Consider boron trifluoride BF 3.
Nitric oxide NO is an example of a stable free radical. Which of the following molecules include a. Which response includes all the molecules below that have a central atom that does not follow the octet rule.
Hydrogen atoms can naturally only have only 2 electrons in their outermost shell their version of an octet and as such there are no spare electrons to form a double bond with boron. Such compounds are called incomplete-octet compounds. Which of the following does not follow octet rule.
BF3 O II and III only O I II III and IV oll only O I II and IV only I and IV only. BF3 does not obey octet rule. Each atom has an electron configuration like that of helium B.
It is pssible to draw a structure in which every atom has an octet. Octet rule simply means that the central atom. BF3 and octet rule.
H20 CO2 CH4 NH3 BF3. Boron hes how Other notable exceptions are aluminum and boron which can function well with six. Less common than hypervalent compounds but by no means rare are species in which an atom does not achieve an octet of electrons.
BF 3 is not following the octet rule. Boron commonly makes only three covalent bonds resulting in only six valence electrons around the B atom. When placed between two atoms the.
Circle the species below that violate the octet rulea PCl5b OH-c BeI2 arrow_forward 1You have made structures of NH3 and H2CO molecules in the Part A of your lab reportBoth NH3 and H2CO molecules have three electron groups around the central atomHowever their molecular geometries are not the same. Which one of the following compounds does not follow the octet rule. Which of the following is the least strong.
Is octet rule valid. The bonding is relatively simple to model with a Lewis structure if we allow each valence level electron in the boron atom to be shared in a covalent bond with. It is less stable than either of the hydrogen atoms by itself D.
The third violation to the octet rule is found in those compounds with more than eight electrons assigned to their valence shell. Hence the correct answer is D. Nitrogen molecule will be achieving octet by sharing three electronsspan.
H2O BF3 CCl4 NH3. Which means it does not follow the octet rule. B has only six valence electrons so it violates the octet rule.
Rigid body is perfectly elastic body b. BCl3 do not obey octet rule It is a electron deficient moleculeAs it share only three electron with chlorine atom. Why does boron trifluoride not follow the octet rule.
Mark the correct statement. What element does not follow the octet rule. The compound actually exists but it is highly reactive that is to say unstable.
Does BF3 have a complete octet. The only possibility for boron is to bond to three hydrogen atoms in which case it forms a compound borane BH3 that does not fulfill the octet rule. Calculating the formal charge on each atom using.
The N atom has an only seven valence electrons so it does not obey the octet rule. It does not obey the octet rule on the nitrogen atom. As it contains 6 electrons outside of the central atom.
The octet rule is a chemical rule of thumb that reflects the observation that elements tend to bond in such a. Molecules having an odd number of electrons around them do not follow the octet rule. While in BF₃ after it formation there are only 6 electrons around the central atom Boron.

Nature Of Covalent Bonds 8 2 Cont D The Difference Ppt Download

Atoms In Second Row Can Have Less Than An Octet Ex Ppt Download

Octet Rule Definition Limitations Of Octet Rule Hypovalent Hypervalent Compounds By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
Which Atom In A Bf3 Compound Has An Incomplete Octet Quora
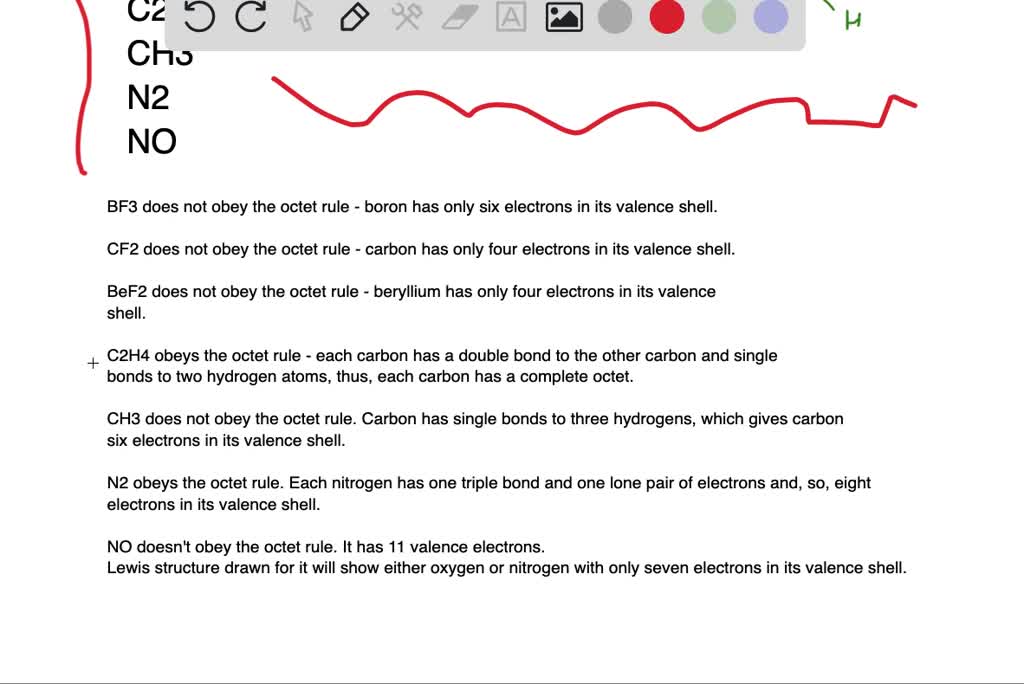
Solved Which Of The Following Molecules Have An Atom That Does Not Obey The Octet Rule Not All Of These Are Stable Molecules A B F 3 B Mathrm Cf 2 C Operatorname Be Mathrm F 2 D Mathrm C 2

In Which Of The Following Molecules Does The Central Atom Not Follow The Octet Rule Youtube

Calculating Molar Masses Teaching Chemistry Chemistry Classroom Chemistry Worksheets

Identify The Atoms In Each Of The Following Compounds Which Do Not Obey The Octet Rule Sf6

Chemical Bonding Although We Have Talked About Atoms And Molecules Individually The World Around Us Is Almost Entirely Made Of Compounds And Mixtures Ppt Download
Using Formal Charges Why Does Boron Not Have A Full Octet Quora
Which Atom In A Bf3 Compound Has An Incomplete Octet Quora

Solved One Of The Following Is Not Obey Octet Rule A Co2 B No3 Co3 2 D Beclz

Solved In Which Of The Following Molecules The Central Atom Chegg Com

Icl4 Lewis Structure Tetrachloroiodide Ion Molecules Lewis Biology
Exceptions To The Octet Rule Video Khan Academy
How Can The Octet Rule Ever Be Broken Like In Bh3 And Bf3 Isn T The Octet Rule For Stability So Why Is Bh3 Even A Compound Why Does It Exist If It

Comments
Post a Comment